 A major reason crop nutrition seems to be getting more complex is due to the swings in commodity prices, which quickly change revenue, input costs and profit margins.
A major reason crop nutrition seems to be getting more complex is due to the swings in commodity prices, which quickly change revenue, input costs and profit margins.
There was a time when fertilizer management only focused on N-P-K. As yields climb, we know have to increase the productivity of N, P and K through the supporting roles of secondary and micronutrients, such as sulfur and zinc all the way through molybdenum.
While we tend to focus on the cheaper inputs, we ask hard questions – like what if copper holds the yield of your wheat crop back and the 50 pounds of nitrogen you applied isn’t productive because it wasn’t supported by $5 worth of copper?
Economics and our biases sometimes interfere with our decisions. Remember: in most instances, balance is better than abundance.
Fertile Soil = Higher Yield, Right?
Improving crop yields and fertility is a start, but not the whole story. The expectation of a fertile soil is that it will share those nutrients with the crop. Fertile soils are generally productive, so there is a correlation – but not always:
- Excesses of some nutrients can interfere with the utilization of good levels of other nutrients.
- Higher rainfall amounts will cause some nutrients, such as potassium, to move down in the soil.
- With dry climates, high evaporation rates will keep cations at the surface.
- Arid areas are often receiving irrigation water. Since the irrigation water generally carries other nutrients, the top of the soil acts as a filter and the soil can take on the properties of the water. The result can be a potassium level that may have you gloating over how fertile the soil is, but could actually limit production.
These factors matter as we manage crop nutrition. We can manipulate levels, but then we’re back to economics to see if productivity will justify the expense.
Let’s get into the details by looking at some of the information report in a soil test analysis.
Cation exchange capacity (or CEC, and pronounced like a short phrase: cat-I-on)
The CEC shows us the nutrient and water holding capacity of the soil. This is the first indicator of the productive capability of a soil. The higher the number, the more water and nutrients it can store.
Soils with all levels of CEC can be productive. Low numbers can be challenging because they need rain or irrigation more often since these sandy soils don’t store much water. But crops root down well in a sandy soil, and with sufficient water, they will reward you with outstanding yields.
Higher CEC soils hold more nutrients and water, providing a buffer between rain events. A low CEC number would be 1, requiring superb management, and a high number might go as high as 50 if a lot of organic matter exists. Typical soils range between 10 and 30. Some tests factor the organic matter into the nutrient holding capacity and with other tests, you need to account for it separately.
Calcium (Ca)
Calcium levels heavily influence soil productivity, and growers sometimes aim for a a Ca base saturation range of 60-75%.
Higher numbers will tie up other nutrients. With a number higher than 75, growers can check if it is tying up phosphorus and crowding out the micronutrient cations like zinc, iron, and manganese. If it is, growers need to allocate budget for adding these inputs.
If it’s a case of a low nutrient base saturation, such as phosphorus or one of the micros, those low levels are related to an excess of something.
Phosphorus is very reactive with calcium and since zinc, iron, manganese and copper are cations, they can be displaced by high levels of calcium. Most commonly, you’d see associated low levels of zinc, iron and manganese . The importance of sulfur is elevated to counter high calcium levels. You aren’t only considering crop needs for sulfur, but also the ‘antidote’ effect it has on the elevated cation level of calcium.
Banding near the root zone is important to limit exposure of the nutrients to reactivity of the high calcium levels. On the other end of the spectrum, at lower than desired calcium levels, below 60%, rhizobia bacteria don’t do their job as efficiently, so lime is needed, especially if growing legumes.
Magnesium (Mg)
Growers can aim for Mg to be between 10 and 20%. Over 20, and compaction is more of an issue. An anaerobic condition can develop under heavy rain or even with severe drought. Under 10%, deficiencies begin to occur and applications should be made. Magnesium is central to chlorophyll development, so it is important to have enough of this nutrient available to your crop.
Potassium (K)
It should be between 3 and 8%. A low C.E.C soil should be in the high side of this range to supply ample amounts. If not, then it needs addressed. Rare instances of levels over 8% can exist and can restrict water infiltration. These high levels would most likely be found in low rainfall areas with high applications of manure or with potassium being brought to the soil through irrigation.
Hydrogen (H)
Any amount of hydrogen present means we are on the acidic side of the pH scale, meaning under 7. The higher the hydrogen number, the more acidic the soil. Aim to keep your soil near the neutral level of 7. As soils become acidic, some nutrients are more readily released, such as iron and manganese.
Often, you see crops that like high levels of iron and manganese grown in acidic soils, such as blueberries. But many beneficial microbes can’t survive in an acidic environment, so generally lime is needed to raise the pH. This is done by adding lime with calcium and/or magnesium, which displaces the hydrogen and brings the pH up. Rain and snow (H2O) bring H to the environment, so acidity slowly creeps back in. Also, various forms of nitrogen can contribute more than others. NH3 and the conversion of urea to NH4, then to NO3 through the nitrogen cycle, contribute to acidity. These forms of nitrogen don’t cause a radical shift in pH, but over-applications do contribute additional hydrogen and creates some acidity.
Sodium (Na)
Sodium mostly comes into play in arid areas where irrigation water is being applied, but can be a factor in low areas of fields where water stands and in areas with a shallow water table. A base saturation of sodium over 2% can limit production when temperatures rise and water is demanded by the plant to cool itself. Sodium holds on to water and can limit its movement into a plant. Generally, elemental sulfur would be used to counter this situation. Also, winter annuals typically do better if sodium issues are persistent. They are grown when transpiration rates aren’t as high, so the competition of sodium for water isn’t as critical. The source of sodium should be identified and treated if possible so that production options remain flexible.
Cation Management
Cation management greatly influences the productive capacity of your soil. Proper balance is important for other nutrient inputs to provide maximum return. Again, calcium in a range of 60-75%, magnesium between 10-20%, potassium between 3 and 8%, hydrogen less than 10% and sodium less than 2% will provide the most consistent yields through a variety of environmental conditions. Exceptions for specific crop reasons and economic limitations of amending soils can create a to manage around problems in this area. A perfect soil doesn’t always make sense. Where possible, it lowers risk of other stresses limiting production, but good production can come from less than ideal soils if they are properly managed.
Looking at Relationships Between the Nutrients
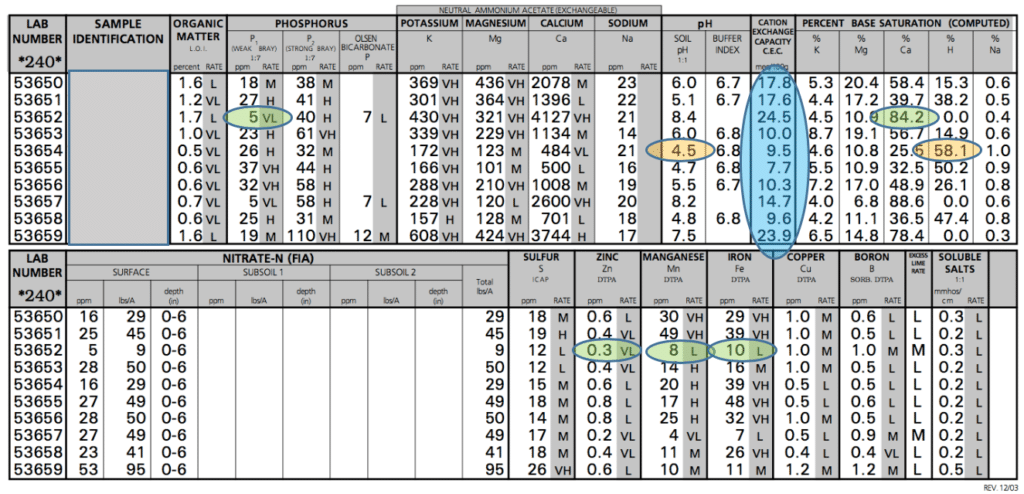
You can see that when looking at a soil test, there are many relationships that exist among nutrients and you shouldn’t just evaluate each nutrient on its own, or address it according to the number listed.
In the example shown, you’d be tempted to look at the weak bray phosphorus (P1) test and apply a very high amount of phosphorus. But if that phosphorus is broadcast, the excess Ca represented by the 84.2% is just going to tie it up, rendering it unusable by the crop you are intending to feed. So stay focused on what the crop will be able to use, and not what you are applying to the soil. Much of the phosphorus that is in the soil on the test with the high Ca will never be used by the crop. The calcium-phosphorus bond is too strong for the plant to be able to extract the phosphorus from the calcium. This just illustrates one of many possible reactions that can limit the ability of a crop to utilize nutrients in the soil. Over time, some of the phosphorus will separate when it rains and a fractional amount will be released.
Micronutrients
Now for another critical area of a soil test, which often growers don’t test for – micronutrients. At the bottom of the test shown, these levels are listed. The balance of the soil significantly impacts micronutrient levels. High levels of Ca, Mg, K and Na can crowd out micros. Often, zinc, manganese, and iron are the most common to have limited availability with excesses of cations. This can easily be seen when crops that have a high demand for these micronutrients are grown on these soils.
Soybeans grown on the soil test with 84.2% calcium would be highly likely to exhibit a chlorotic look because iron and manganese are limited in availability – as shown on the example soil test. Soybeans have a higher need for these nutrients than a crop like corn. Citrus is another crop that demands a lot of iron and manganese and would suffer in this soil.
Building Your Crop Nutrition Management Plan
There are a variety of reasons to routinely test your soil:
- The overview helps you prioritize investments into your asset of land.
- If you own the land, you can perform longer-term fixes, like implementing some practices to correct imbalances.
- If your asset is rented, the overall soil test can help with making these decisions, or even having a conversation with the landowner to ensure you can recover your investment into their asset.
Making Decisions
Decisions regarding crop nutrition can be complex as yield expectations rise and economics remain challenging. AgroLiquid takes measures to mitigate the impact of imbalances that exist by protecting our nutrition from many of the reactions that can occur.
Thinking about fertilizer in terms of how much is used by the plant rather than how much is applied to the soil is a critical step. A complete soil test is a good indicator of how much efficiency can be expected with applied crop nutrition. AgroLiquid’s unique protection improves efficiency dramatically. Couple that with a staff that has a thorough understanding of the nutrient relationships in the soil and you will receive a value that goes beyond the return on your investment.
Future investments into fertilizer should begin with a soil test; they should end with a decision to get the most value out of your fertilizer dollar. Contact your AgroLiquid representative to get started.

